Sartorius Webinar - Optimizing Freeze & Thaw Processes: Choosing the Right Tools for Cryopreservation Success
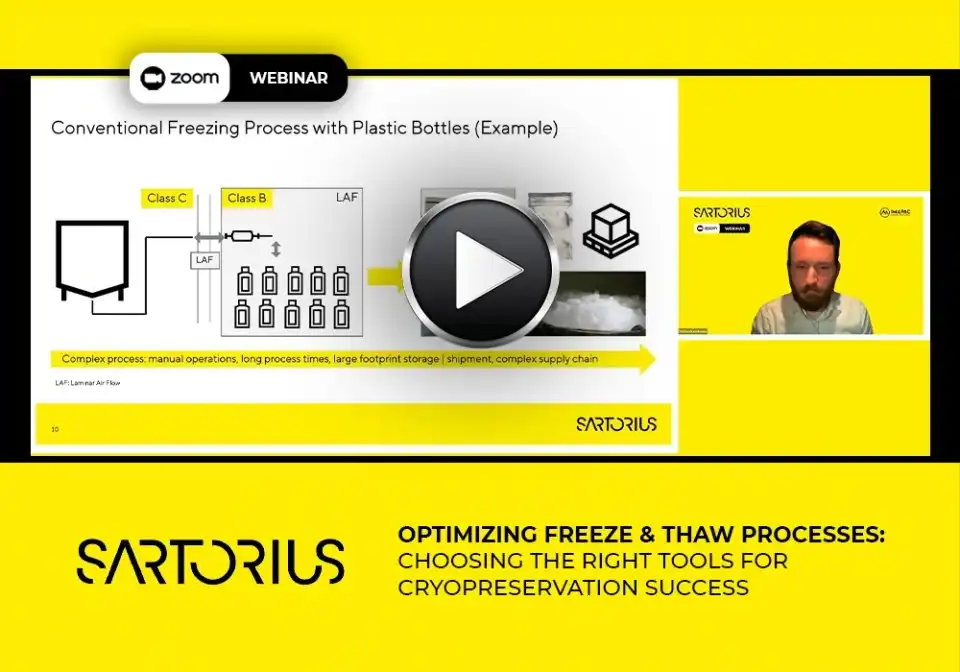
The freeze and thaw (F&T) step in bioprocessing is critical to product integrity, sterility, and scalability. In this webinar, we’ll guide you through selecting the right consumables and hardware for your F&T process, considering molecule characteristics, batch volume, and facility constraints. We’ll compare bottles vs. single-use bags and demonstrate why bags are the preferred option in modern cryopreservation strategies. Learn how Celsius solutions improve handling and sterility, and explore how to quantify benefits using our Total Cost of Ownership (TCO) tool. Whether you're maintaining legacy processes or preparing for a transition, this session will equip you with the knowledge to make informed, confident decisions.
Speakers:
Stefan Woodhouse, Product Manager Freeze and Thaw, Sartorius
Niveditha Shetty, Product Excellence FMT Asia, Sartorius
Download the Full White Paper Here!
DownloadDownload the Full e-Book Here!
DownloadDownload the Full Interview Here!
DownloadOther
Explore other news and updates in the biopharmaceutical industry.
Subscribe For News Updates
Subscribe to the IMAPAC Newsletter to stay informed of the latest news in the biopharmaceutical industry.



%20(2).png)